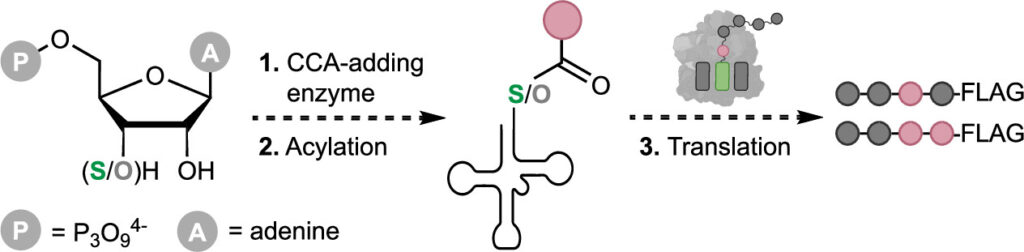
C-GEM researchers found that a single atom change on tRNA—O to S on the 3′ terminal adenosine—does not hinder regular translation. Changing the 3’ oxo-ester on tRNA to a more reactive thioester had the potential to impact translation positively or negatively at multiple steps in the translation cycle. This paper shows that not only can amino-acyl tRNA synthetases and Flexizymes acylate SH-tRNAs, native translation machinery can also utilize SH-tRNAs to incorporate canonical and non-canonical monomers. These findings demonstrate the potential plasticity of the ribosomal active site, and advance C-GEM’s goal of expanding ribosomal chemistry to synthesize new-to-nature biopolymers.
This work involved contributions from the Schepartz lab, Miller lab, and Cate lab.