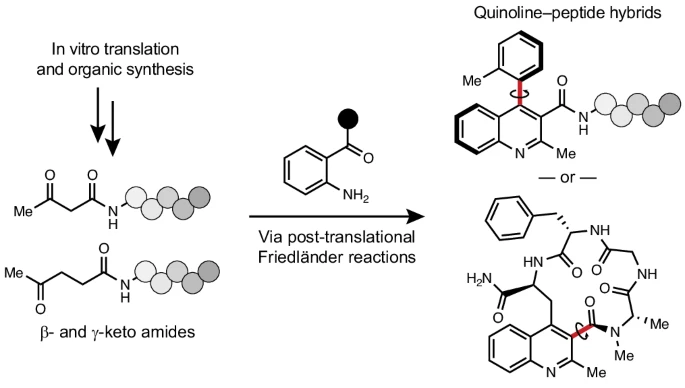
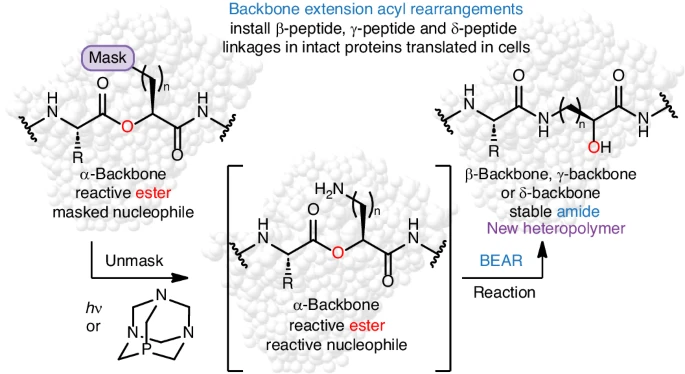
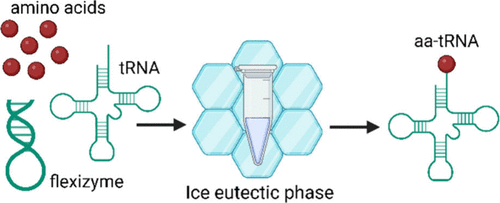
C-GEM Represents at 2026 Peptides GRC
Left: (left to right) Christopher Alabi (Senior Investigator, Cornell), Abhishek Chatterjee (Senior Investigator, Boston College), Alex Solivan (graduate student, UC Berkeley), Taylor Dover (graduate student, Yale), Meghna Bajaj (graduate student, Cornell), Carly Schissel (Assistant Professor, Penn State, former C-GEM postdoctoral scholar).Right: Meghna Bajaj holding her… Read More »C-GEM Represents at 2026 Peptides GRC