Aminoacyl-tRNA synthetases (aaRSs) catalyze the condensation of ɑ-amino acid monomers with tRNA. The resulting aminoacyl-tRNAs are used as substrates by the ribosome to generate natural sequence-defined bio-polymers. The ribosomal synthesis of non-natural sequence-defined bio-polymers demands aaRS enzymes for non-natural monomers. Current aaRS engineering platforms rely on the ribosomal translation of a reporter gene and have limited utility for monomers with low reactivity in wild type ribosomes.
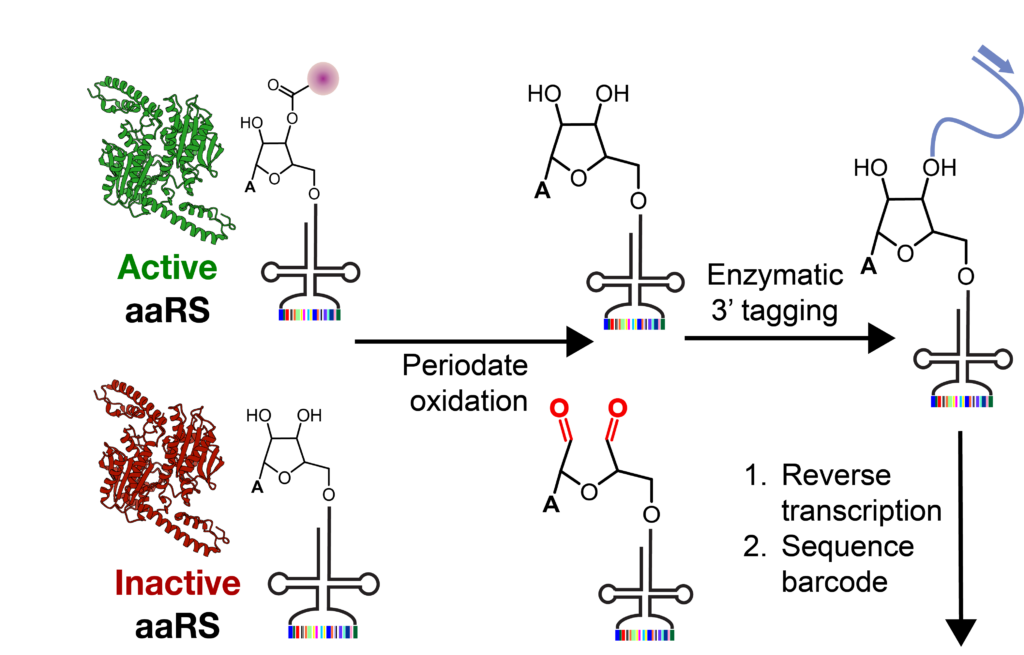
This paper describes START, the first aaRS engineering platform that evolves the enzyme for only tRNA charging, not charging plus translation efficiency. In START, each distinct aaRS mutant is linked to a cognate tRNA containing a unique sequence barcode. Acylation by an active aaRS mutant protects the corresponding barcode-containing tRNAs from an oxidation reaction that damages the 3′-termini of the uncharged tRNAs. Sequencing of the surviving barcode-containing tRNAs reveals the identity of the aaRS mutants that acylated the correlated tRNA sequences. The efficacy of START was demonstrated by identifying novel and cell-active mutants of the Methanomethylophilus alvus pyrrolysyl-tRNA synthetase from a naïve library.
Check out the paper: https://pubs.acs.org/doi/10.1021/acscentsci.3c01557