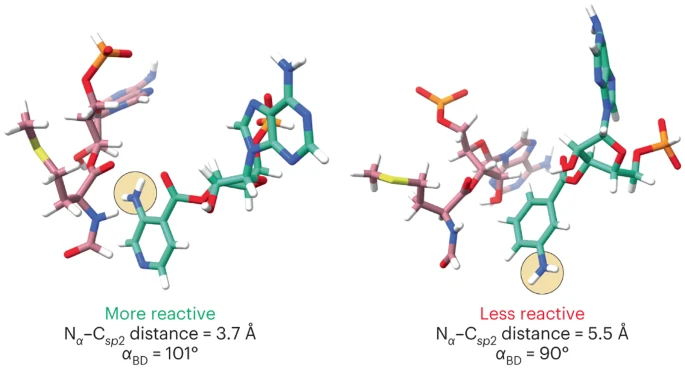
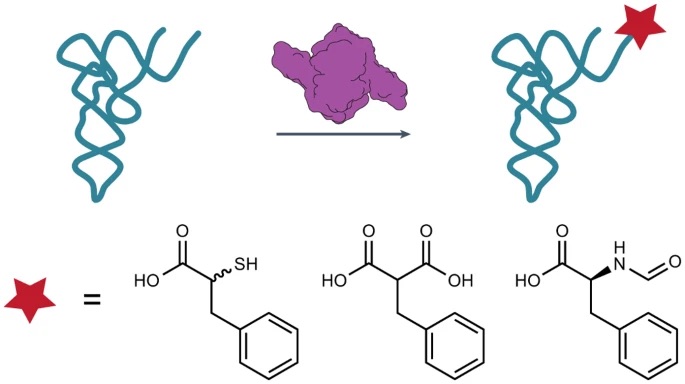
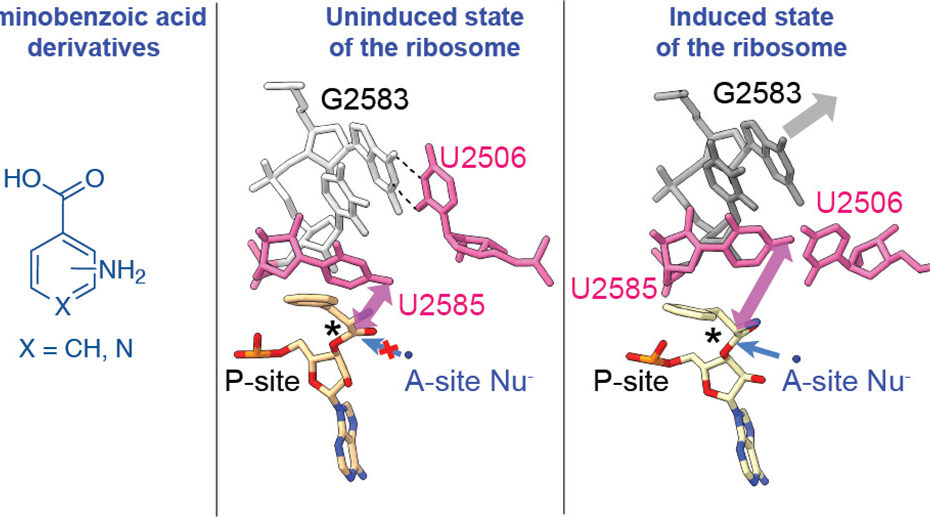
New paper: Atomistic simulations of the E. coli ribosome provide selection criteria for translationally active substrates
There is great current interest in exploiting ribosome chemistry to generate sequence-defined chemical polymers that extend beyond L-⍺-amino acids to new backbone modifications and polymerization chemistries. Although the E. coli ribosome tolerates certain non-L-⍺-amino acids in vitro, few structural insights are available, and the boundary… Read More »New paper: Atomistic simulations of the E. coli ribosome provide selection criteria for translationally active substrates